User Guide
GlyDB is the largest and most comprehensive glycoside database currently available. It contains nearly 160,000 glycoside molecules sourced from five different databases. The database includes information on both the aglycone and sugar moieties of these glycoside molecules. If users discover any errors, we welcome feedback via email at yangruofei@webmail.hzau.edu.cn.
1. Browser Pages
Browse Table
The browsing of moleculars is divided into six modules, natural glycosides, drug glycosides, synthetic glycosides,active glycosides, aglycones, sugars. Glycoside browsing is divided into two ways, table browsing and card browsing. The information for table browsing includes molecular structure diagram, ID, AlogP, MolWeight, Formula, Smiles. Click on the molecular structure picture or ID to view the details page of the glycoside. There are buttons to turn pages at the bottom of the page.

(Browser Table)
Browse Card
The information for card browsing includes molecular structure diagram, ID, AlogP. Click on the molecular structure picture or ID to view the details page of the glycoside. There are buttons to turn pages at the bottom of the page

(Browser Card)
Detail information
The details page for a Glycoside shows its comprehensive details. The information is divided into four modules, which are glycosides, properties, aglycones, and sugars. The aglycone module is the aglycon information after deduplication, and the complete information is displayed in the glycoside module. Click the picture or ID of aglycon to jump to the details page of aglycone, and click the ID of sugar to jump to the detail page of sugar.
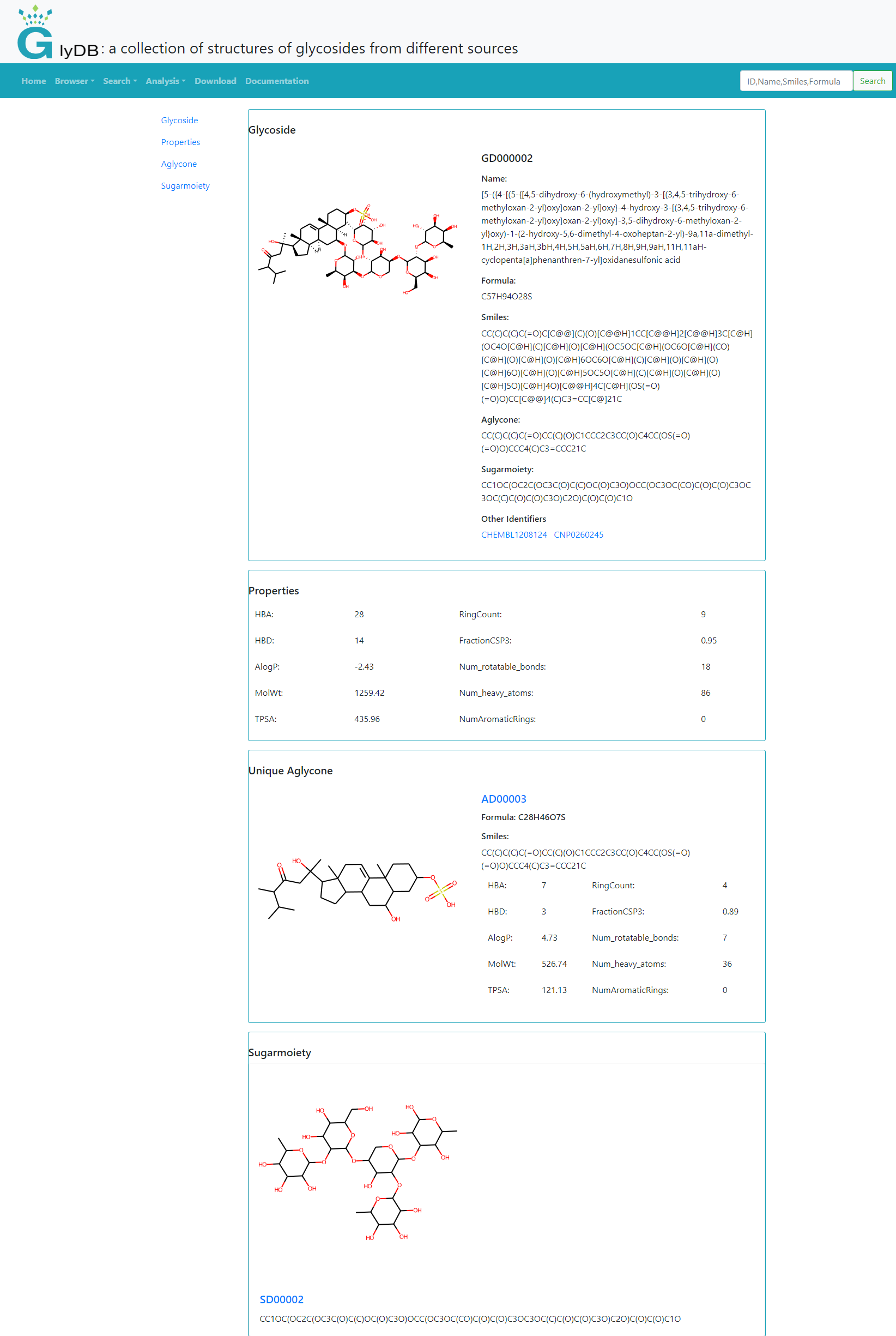
(Detail Information)
2. Search Pages
The GlyDB database provides four search modes: glycosides search, aglycones search,sugars search and simple search.
Glycosides search
Glycoside's search supports input structure search and form search. When the user does not draw a structure on the left, only the form search is used by default. Structure search is subdivided into exact search, substructure search and similarity search. Users can draw the structure themselves, or right-click to paste the structure of the molecule. An exact search refers to an exact match that includes stereoisomeric information. For substructure searches, users can draw the substructure of a compound to retrieve compounds that contain that specific substructure. For similarity searches, users can draw the structure of a compound and set a similarity threshold to retrieve compounds similar to the drawn structure. The form search provides molecular formula, molecular weight and other compound properties for users to search. It is recommended that users refer to the examples below the input box for input.
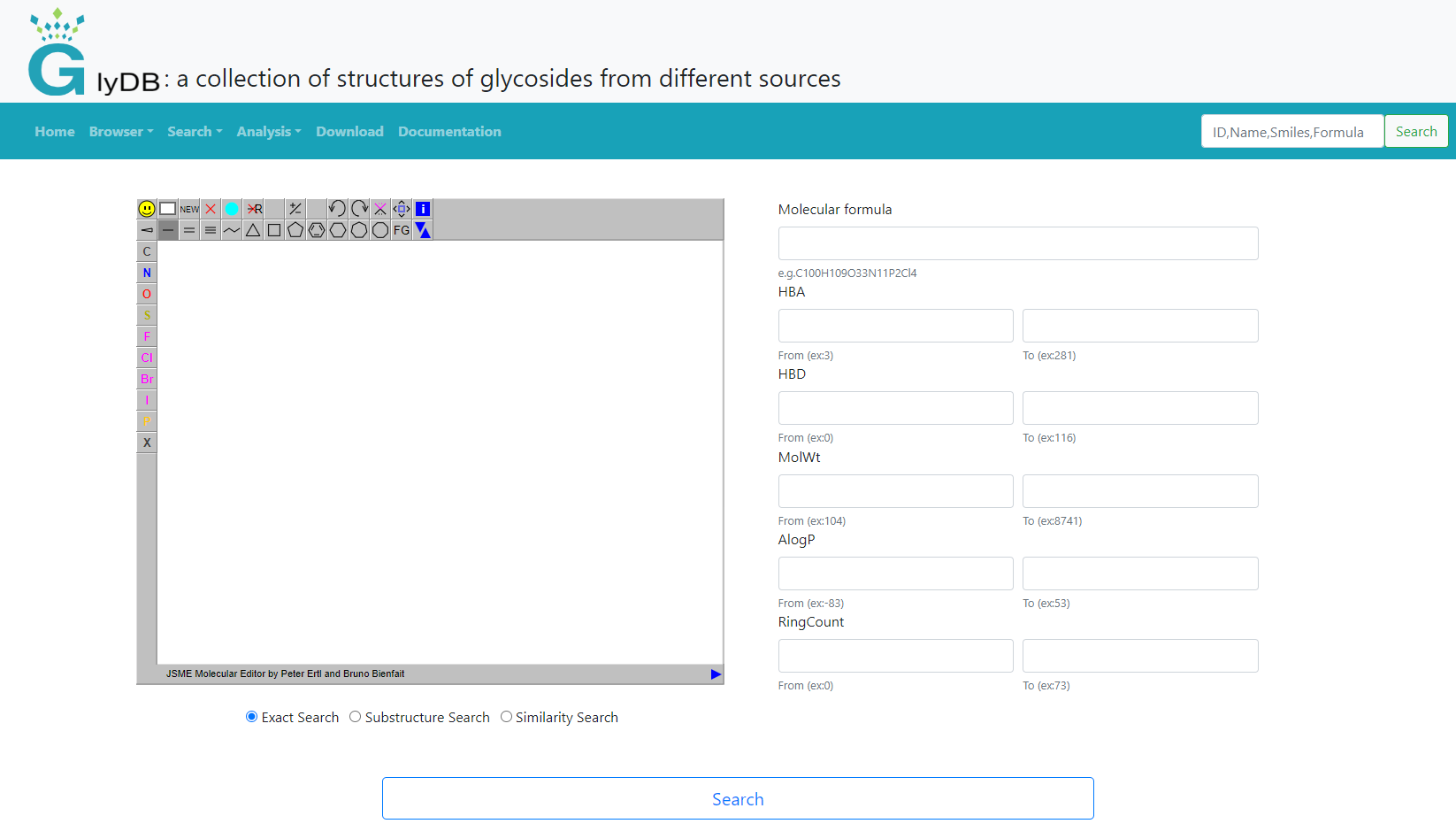
(Glycosides search)
Aglycones search
The aglycon search supports input structure search and form search. When the user does not draw a structure on the left, only the form search is used by default. Structure search is subdivided into exact search, substructure search and similarity search. Users can draw the structure themselves, or right-click to paste the structure of the molecule. An exact search refers to an exact match that includes stereoisomeric information. For substructure searches, users can draw the substructure of a compound to retrieve compounds that contain that specific substructure. For similarity searches, users can draw the structure of a compound and set a similarity threshold to retrieve compounds similar to the drawn structure. The form search provides aglycon ID, aglycon Smiles, molecular formula, and molecular weight and other compound properties for users to search. It is recommended that users refer to the example below the input box to input.
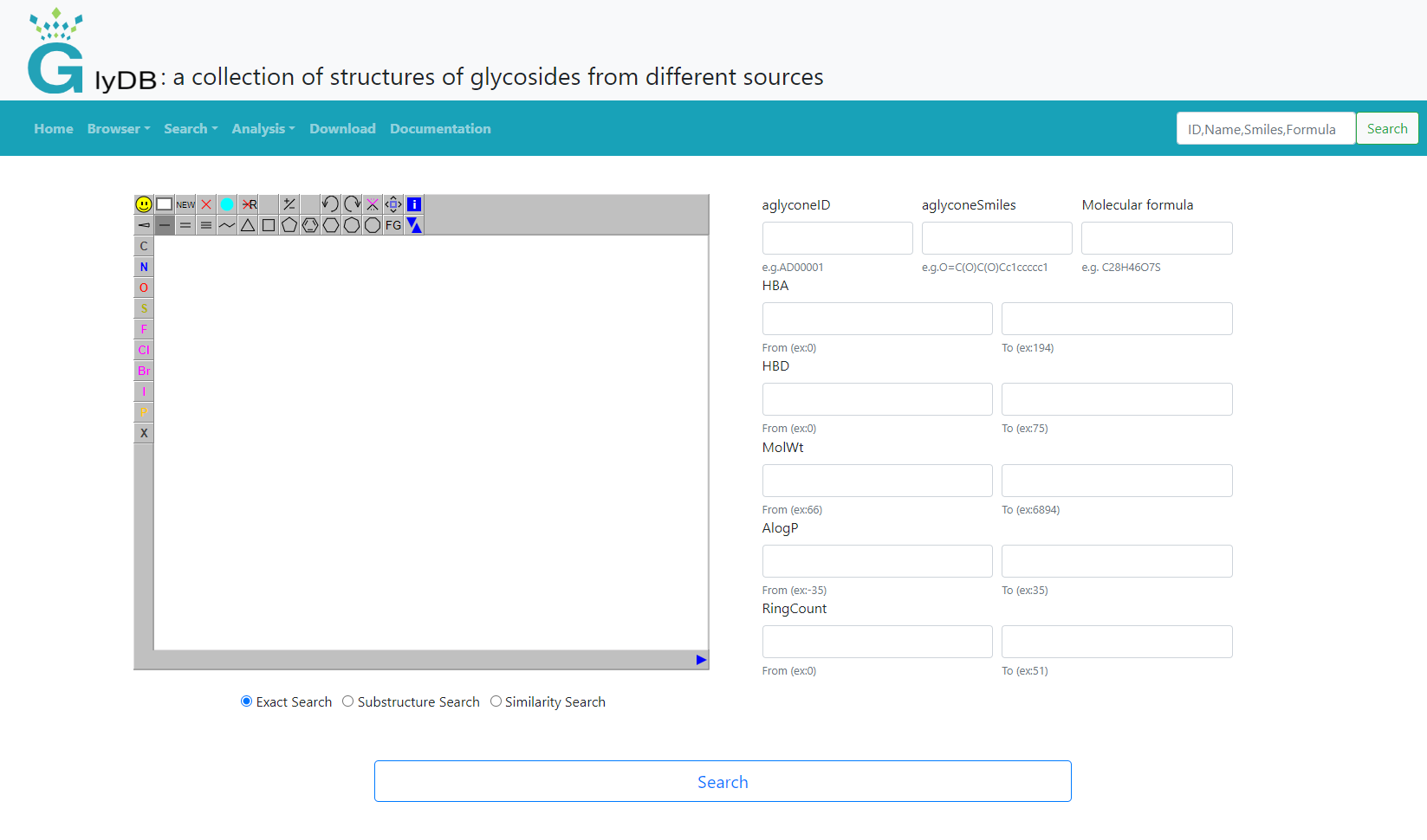
(Aglycones search)
Sugars search
Glycan search supports input structure search and form search. When the user does not draw a structure on the left, only the form search is used by default. Structural search is subdivided into exact search and substructure search. Users can draw the structure themselves, or right-click to paste the structure of the molecule. An exact search refers to an exact match that includes stereoisomeric information. For substructure searches, users can draw the substructure of a compound to retrieve compounds that contain that specific substructure. The form search provides glycan ID and glycan Smiles for easy retrieval by users. Users are advised to refer to the examples below the input box for input.
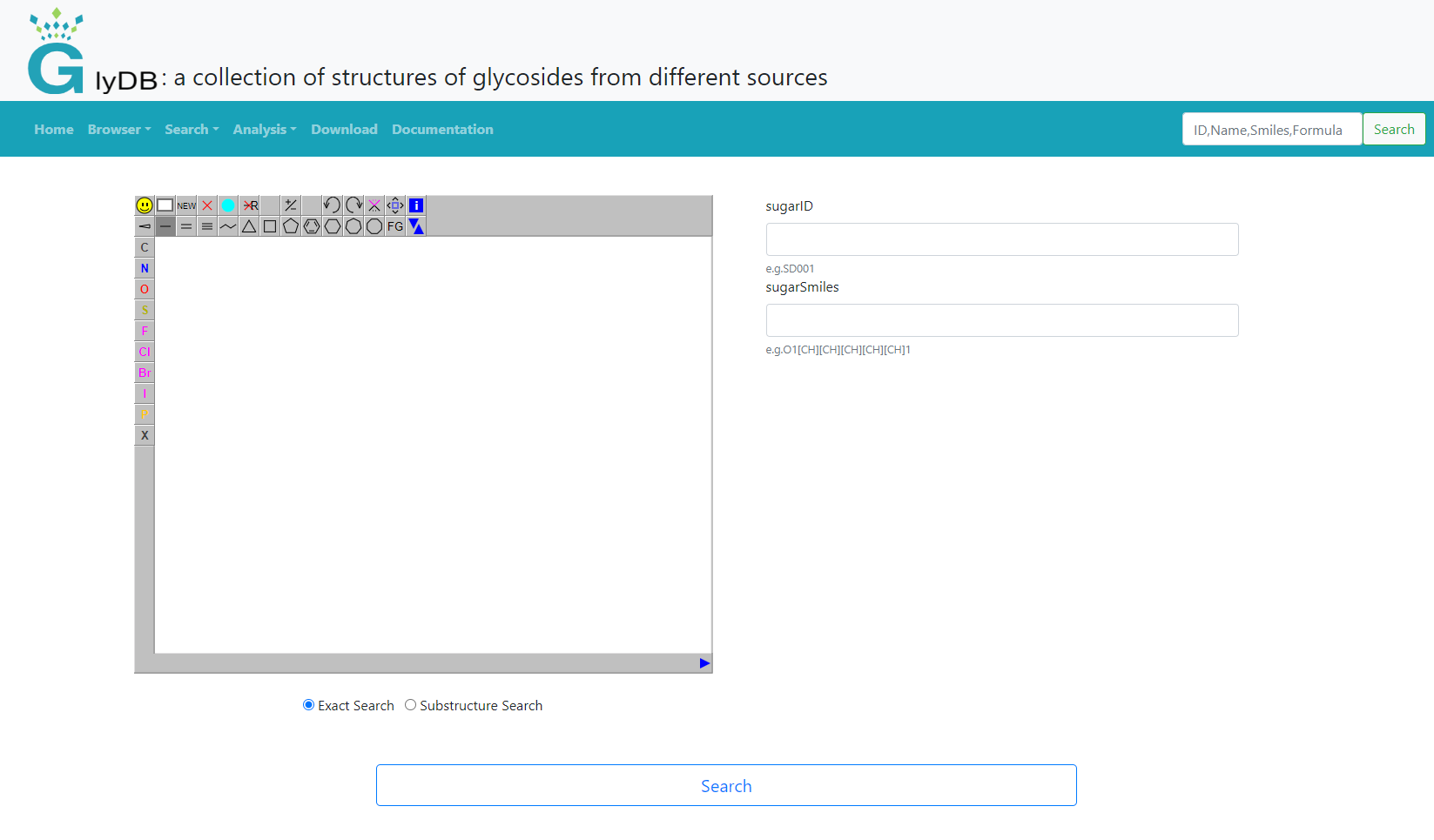
(Sugars Search)
Simple search
Simple Search is located at the top right of all pages. Simple search only supports user input of glycoside ID, Smiles, name, molecular formula.
3. Analysis Pages
The Analysis page includes Scaffold Analysis and Target Analysis.
Scaffold Analysis
The skeleton analysis is divided into four modules, which are the skeleton analysis of natural glycosides, the skeleton analysis of drug glycosides, the skeleton analysis of synthetic compounds, and the skeleton analysis of active compounds. Click on different modules to see different types of skeleton analysis results. All scaffolds are aglycon scaffolds. The number below each scaffold ID indicates the number of glycosides containing the aglycon scaffold. Click to view the corresponding glycoside.
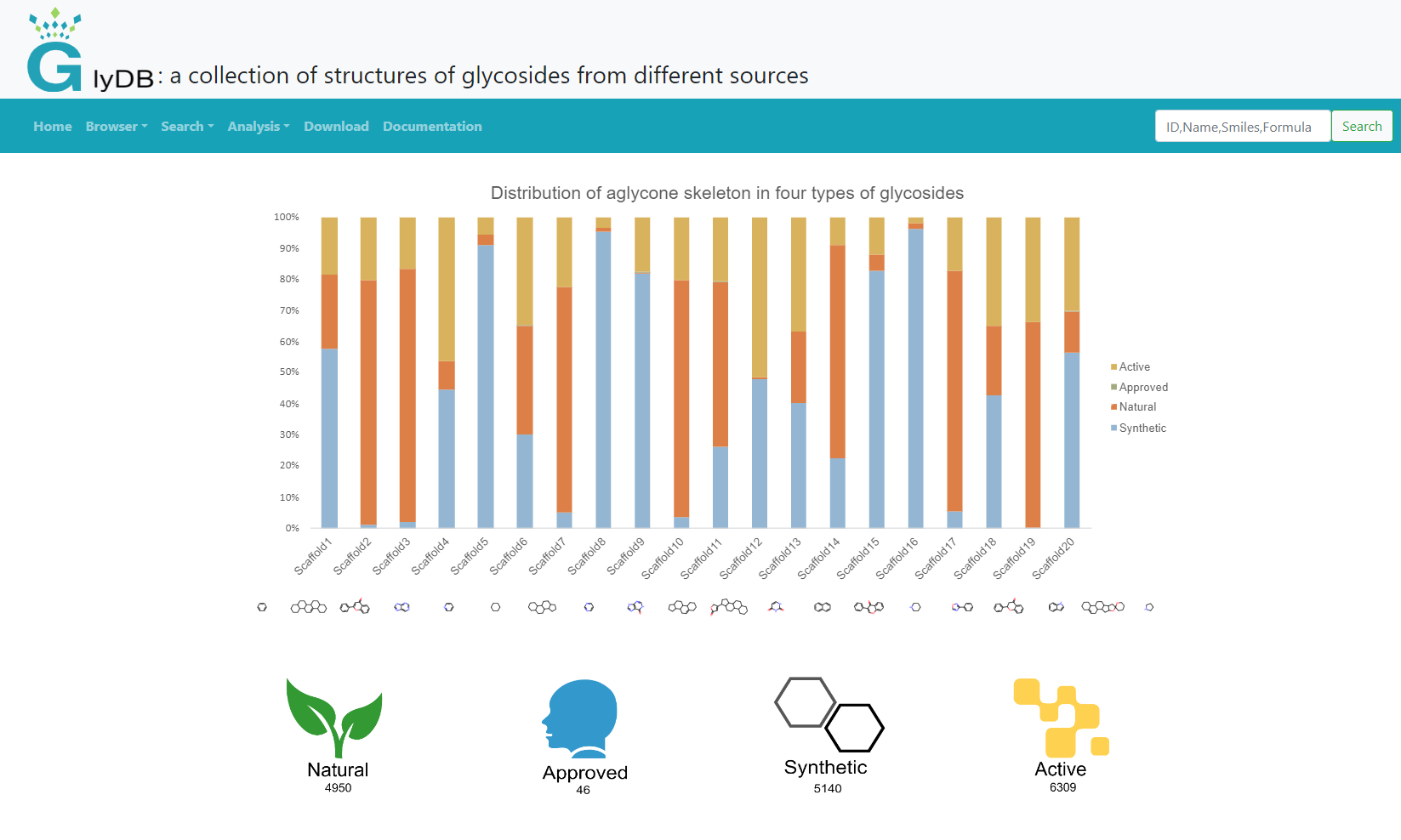
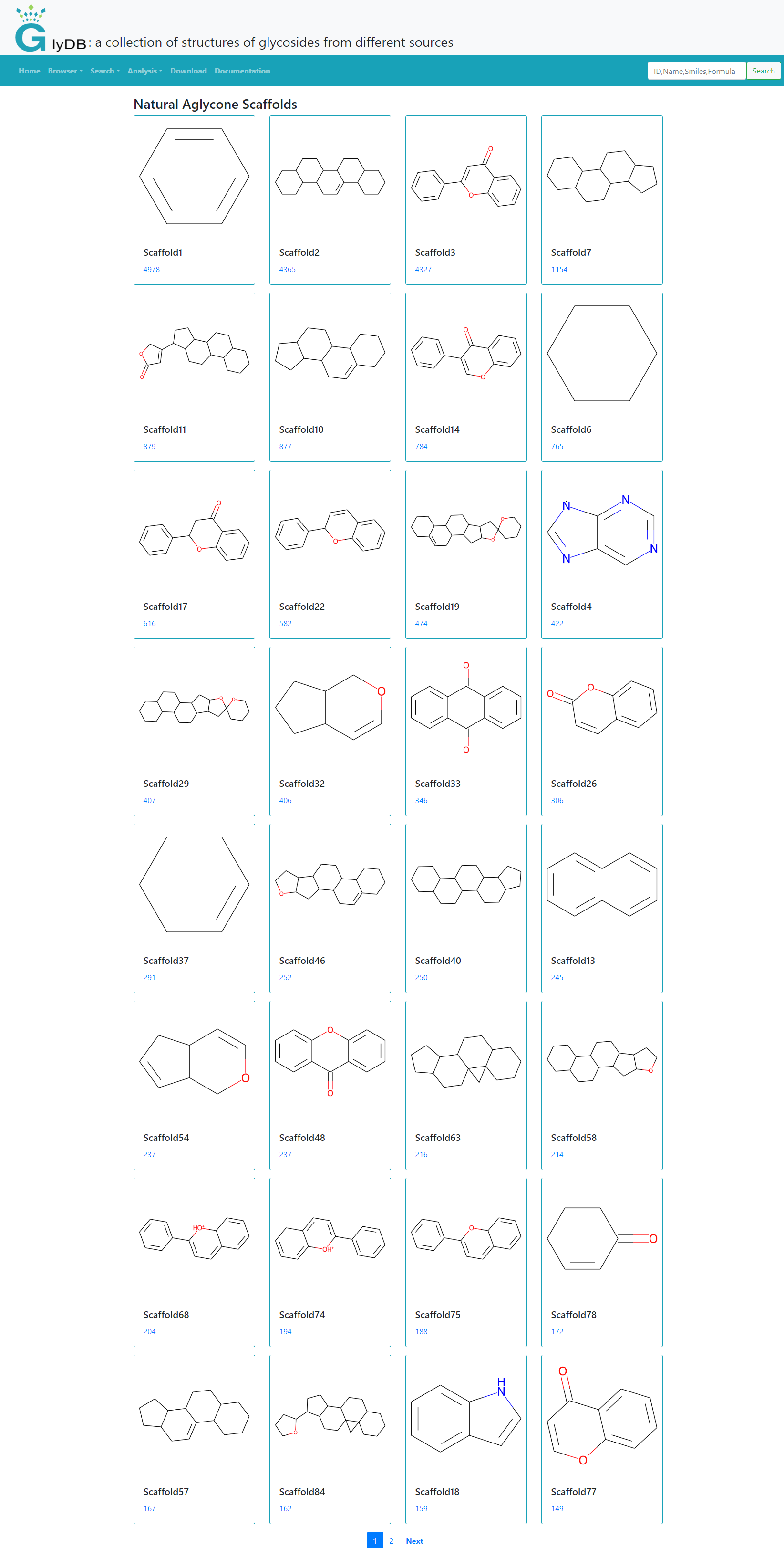
(Scaffold Analysis)
Target Analysis
The target analysis module provides the target type, name, organism, and the number of glycoside compounds corresponding to the target. Click on the target ID to see the glycoside corresponding to the target, the link to the corresponding CHEMBL database, and graphically display the number of glycoside compounds and the proportion of non-glycoside compounds.
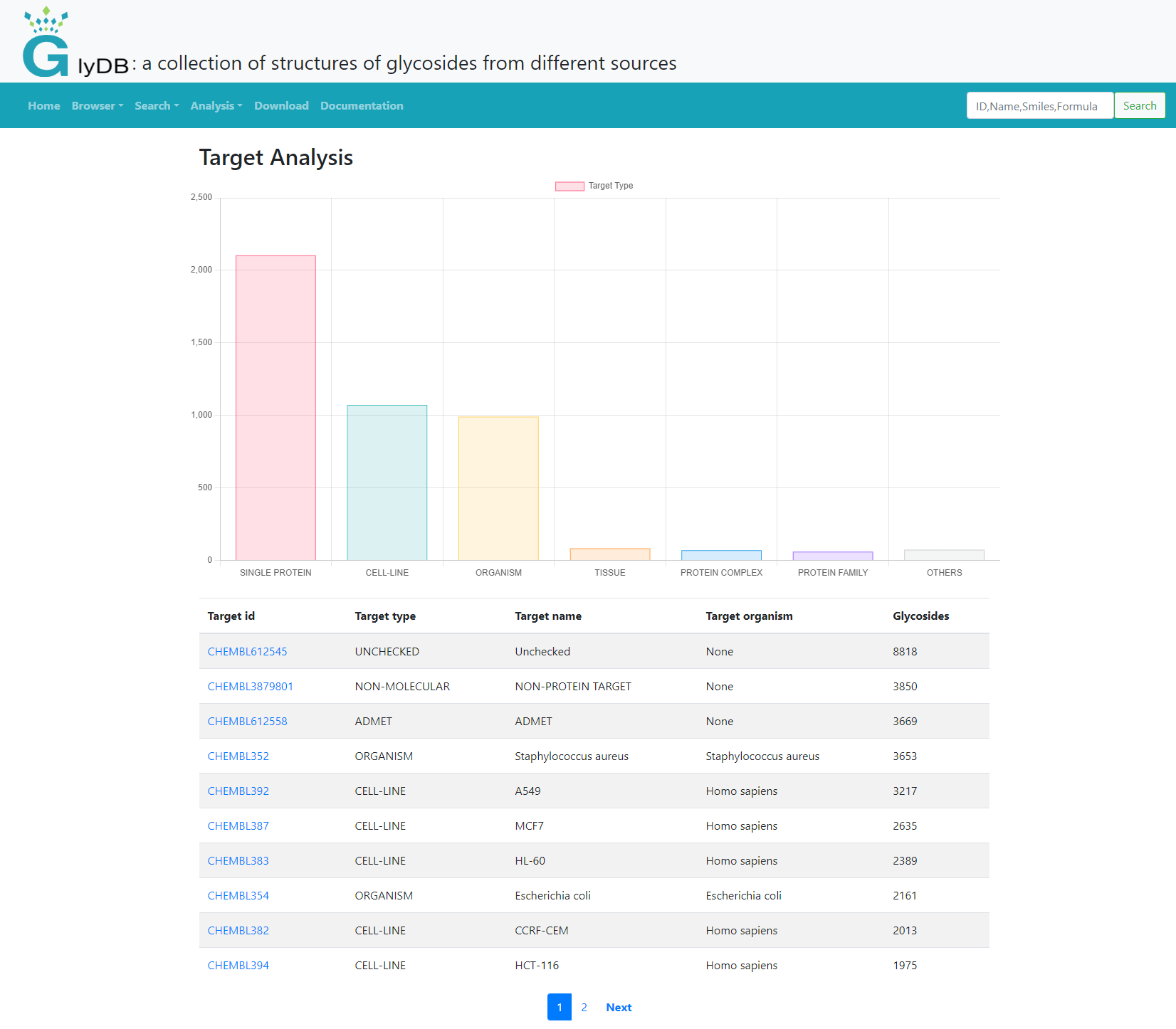
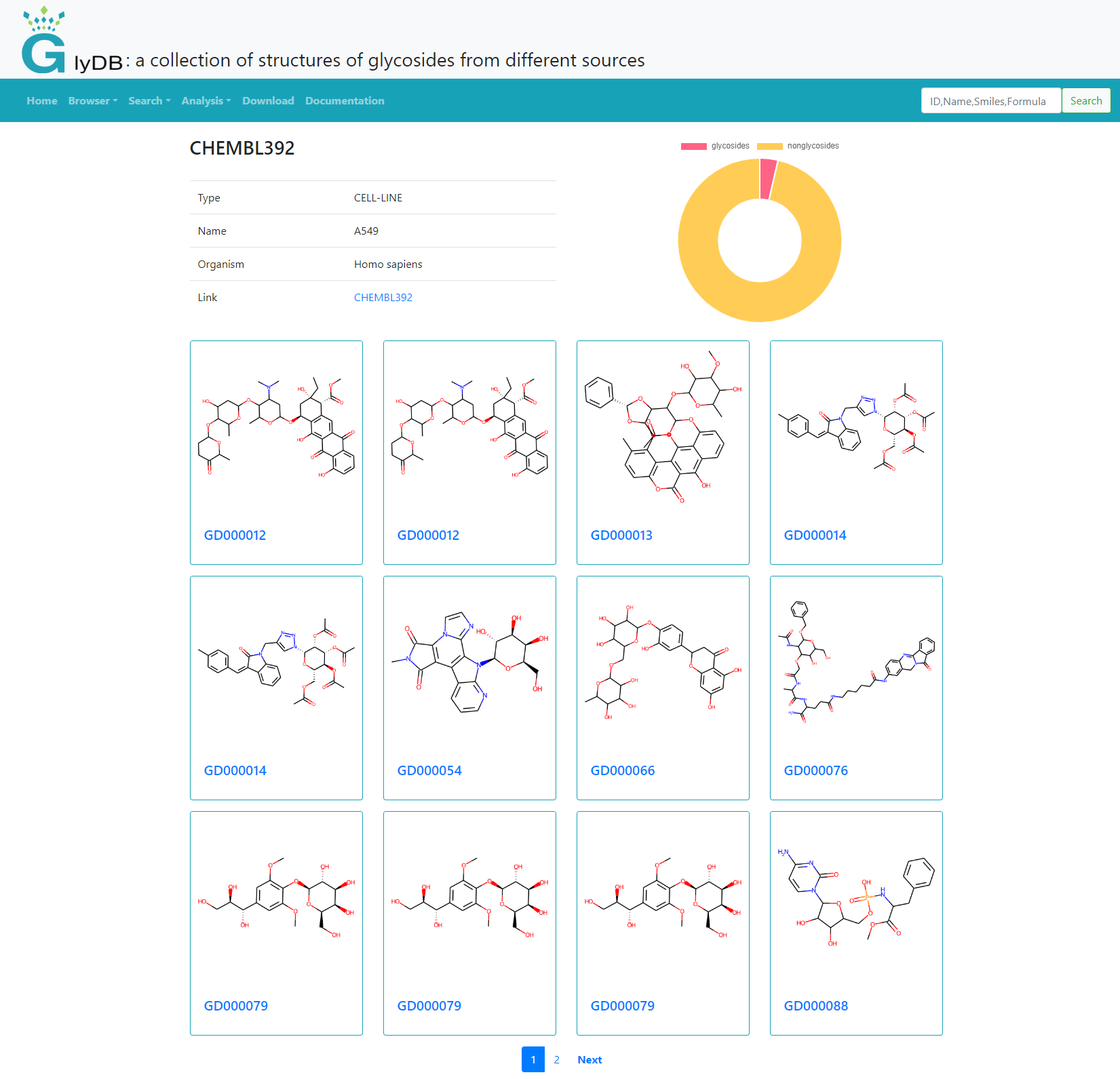
(Target Analysis)
4. Download Pages
The Download page provides the download of ID and structure information (Smiles) of glycosides, aglycones, and glycosyl groups in the GlyDB database
(Download Page)